patent holders signed agreements with the MPP
products licensed to the MPP
active and ongoing product development projects have led to
regulatory filings for HIV products
and
filings for hepatitis C medicines with stringent regulatory authorities
generic manufacturers and product developers sublicensed from the MPP
Generic products
facilitated by the MPP have been
distributed in
136 countries,
providing treatment to more than
22 million
patient-years from
January 2012 to
December 2018
MPP licences
have generated
USD 1.06 billion
in global health savings
through the procurement
of
more
affordable
quality-assured medicines
from MPP generic partners
through
an average price
reduction of
73%
relative to
originator price
Download
this chapter
Our mission is to increase access to, and facilitate the development of, life-saving medicines for low- and middle-income countries (LMICs) through an innovative approach to voluntary licensing and patent pooling. We work with a range of partners – civil society, international organisations, industry, patient groups and governments – to prioritise and license novel and existing medicines and health technologies for people in these countries.
Our vision is a world in which people in need in LMICs have rapid access to effective and affordable medical treatments and health technologies.
In May, the MPP launched its new strategic direction for 2018–2022, setting targets for improving access to medicines for people living with HIV, hepatitis C and tuberculosis in LMICs. Based on the findings of a feasibility study, the plan also recommends the expansion of the MPP model to patented medicines with high medical value, starting with small molecules on the World Health Organization Model List of Essential Medicines (WHO EML).
More than 20 million people living with HIV in LMICs are treated with MPP-licensed antiretrovirals.
The MPP has licensed patented medicines that are on the WHO EML or are likely to be added in the future.
Curative, pan-genotypic hepatitis C treatments are available for ≤ USD 50 per person from quality-assured suppliers in licensed countries.
The MedsPaL database incorporates up-to-date reliable intellectual property status information on all patented essential medicines for all LMICs.
Shortened all-oral regimen with the potential for use in drug-resistant and drug-susceptible tuberculosis is licensed to the MPP.
Of the 36.9 million people living with HIV globally in 2017,
21.7 million had access to antiretroviral treatment,
an increase of 2.3 million since 2016 and up from 8 million in 2010.1
Of the 36.9 million people living with HIV globally in 2017,
21.7 million had access to antiretroviral treatment,
an increase of 2.3 million since 2016 and up from 8 million in 2010.1
[2017]
PEOPLE LIVING
WITH HIV,INCLUDING
1.8M CHILDREN
adults
children
MISS OUT ON HIV TREATMENT, OF WHOM THE VAST MAJORITY LIVE IN LOW- AND MIDDLE-INCOME COUNTRIES
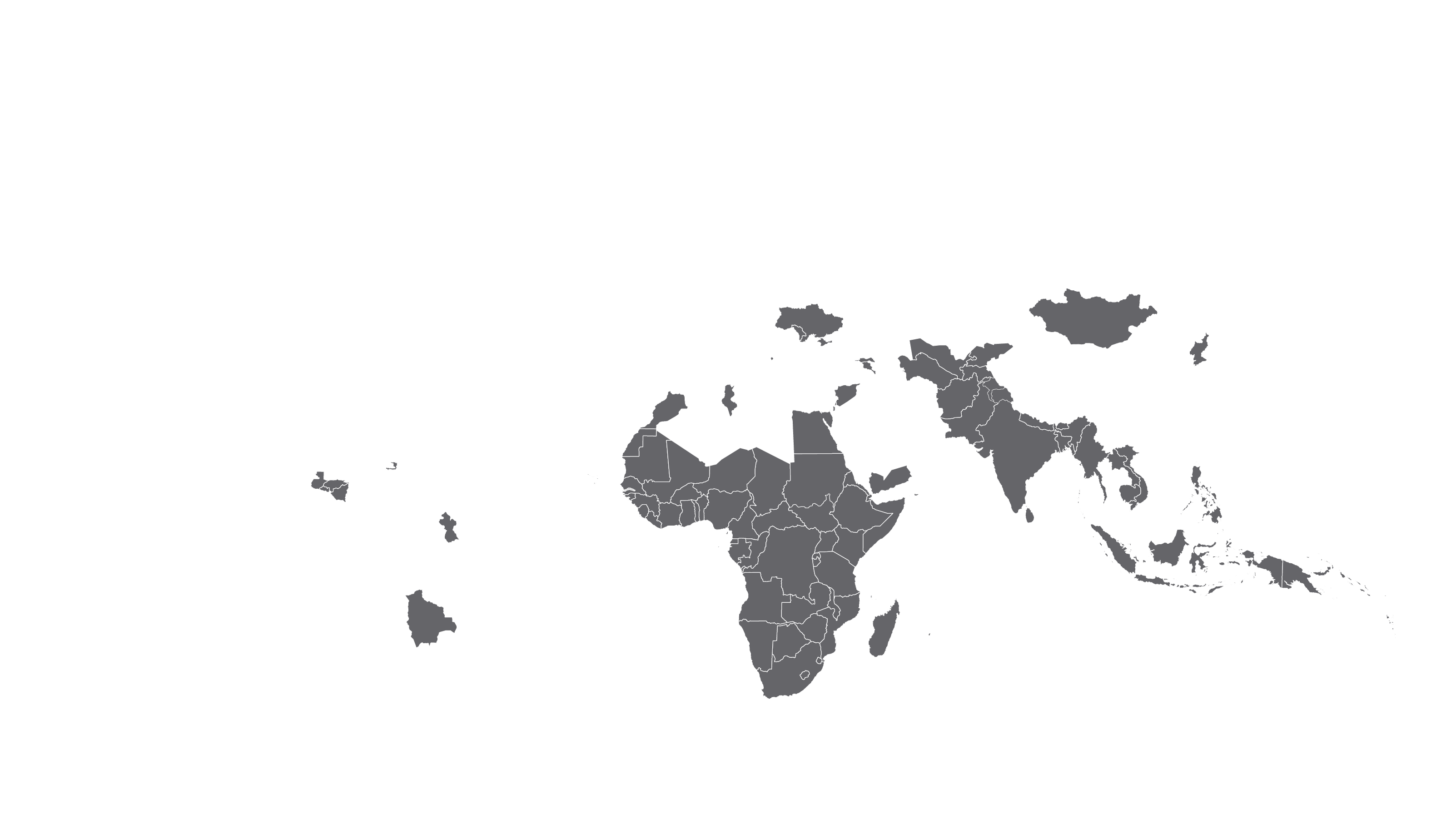
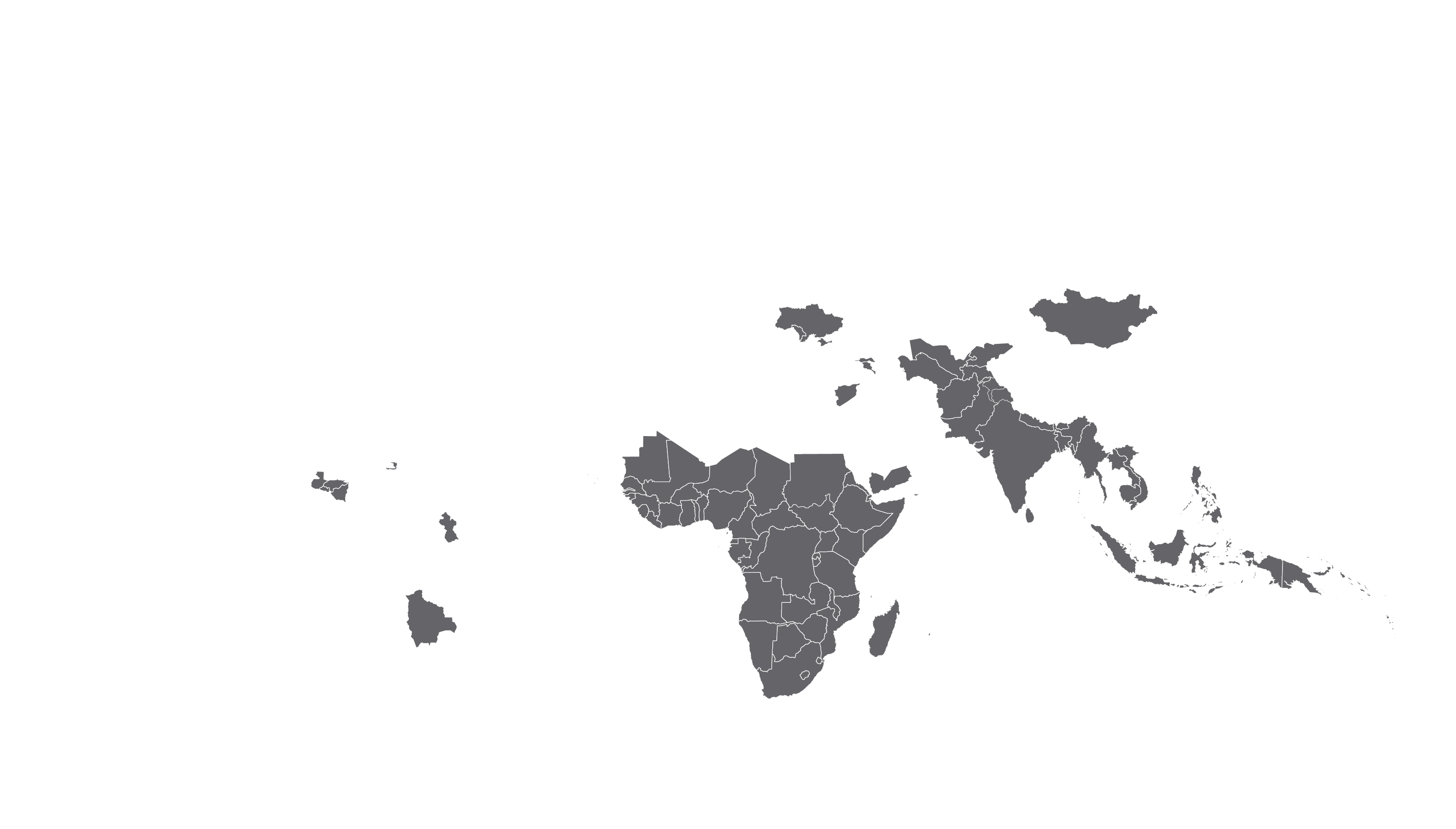
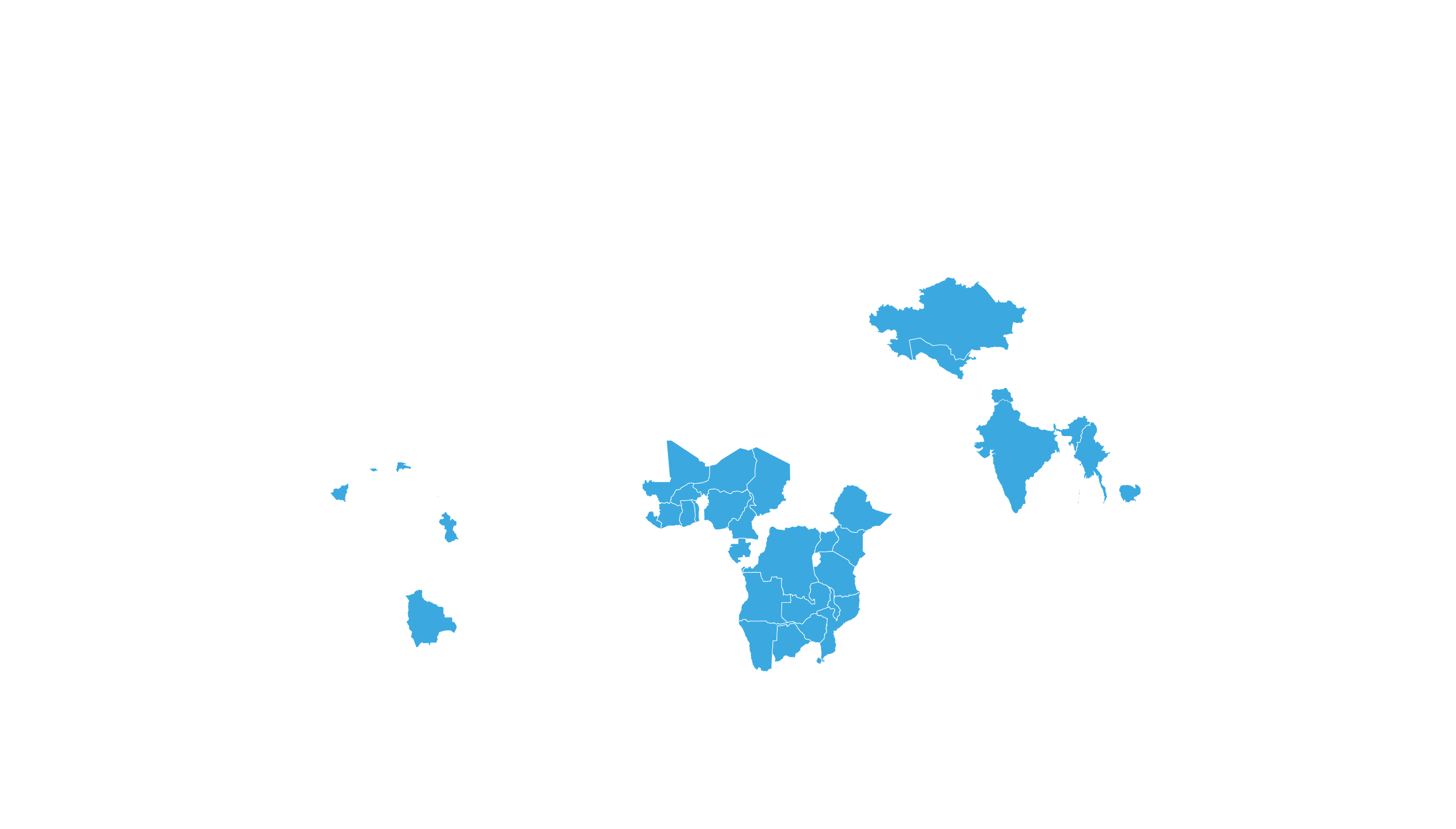
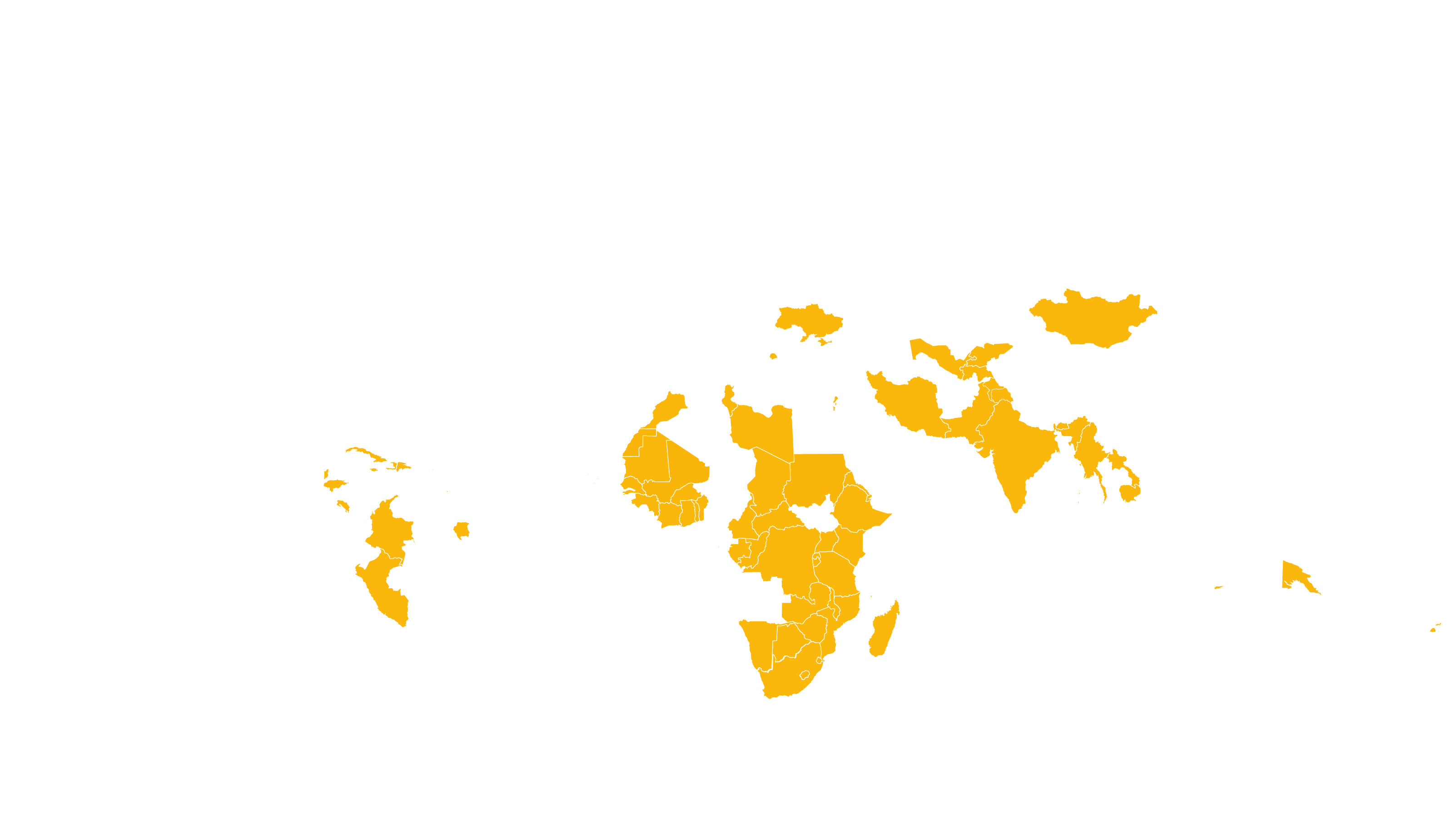
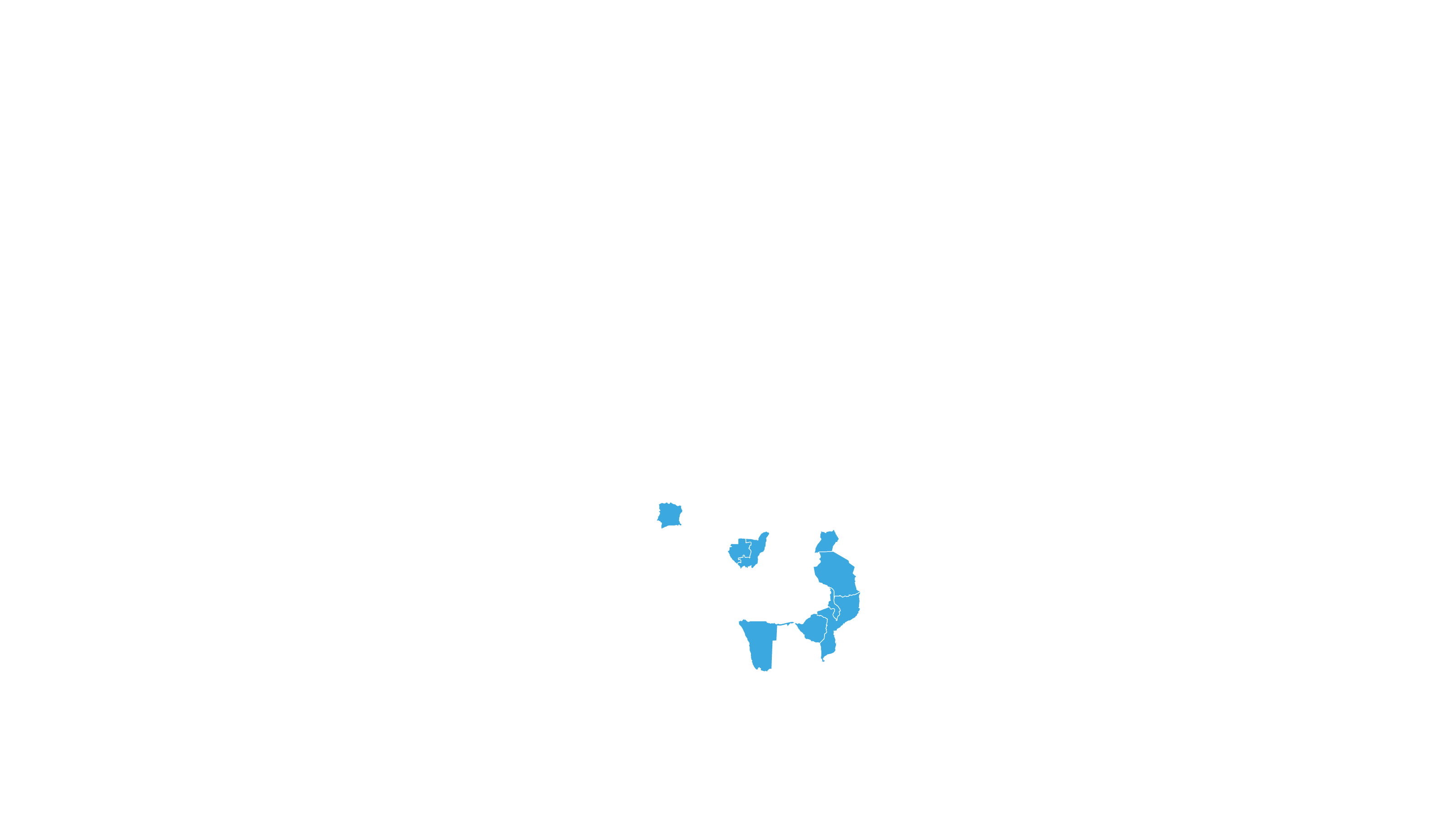
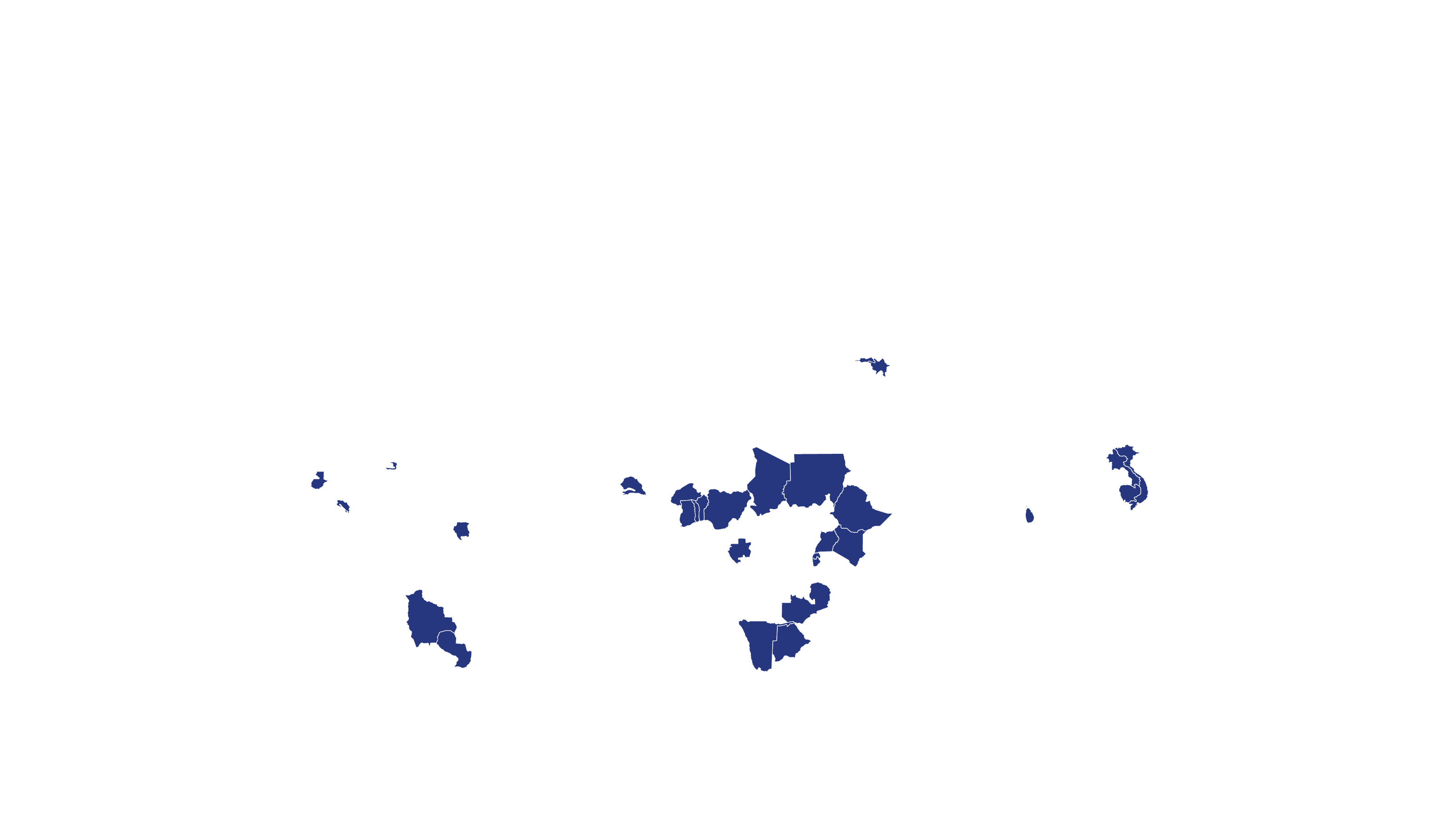
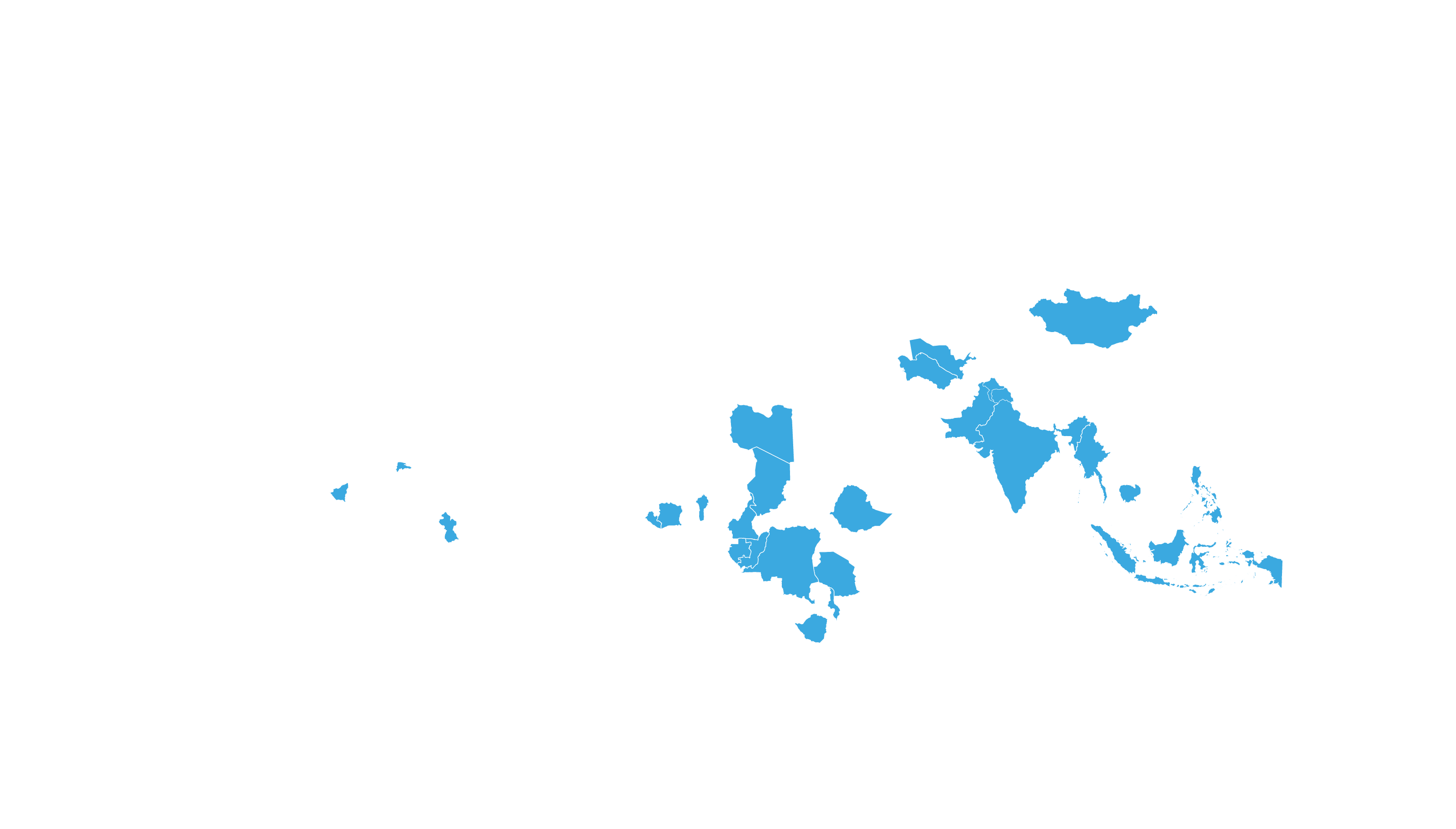
See how MPP's work is increasing access to HIV medicines in low- and middle-income countries - world map visualisation.
Choose product:
dolutegravir (DTG) 50mg
tenofovir disoproxil/lamivudine/ dolutegravir (TDF/3TC/DTG – TLD)
tenofovir alafenamide/ emtricitabine/dolutegravir (TAF/FTC/DTG)
atazanavir/ritonavir (ATV/r)
lopinavir/ritonavir (LPV/r)
lopinavir/ritonavir (LPV/r) paediatric
Choose status:
COVERED TERRITORY
94
COUNTRIES
FILED IN
22
COUNTRIES
APPROVED IN
19
COUNTRIES
SOLD IN
56
COUNTRIES
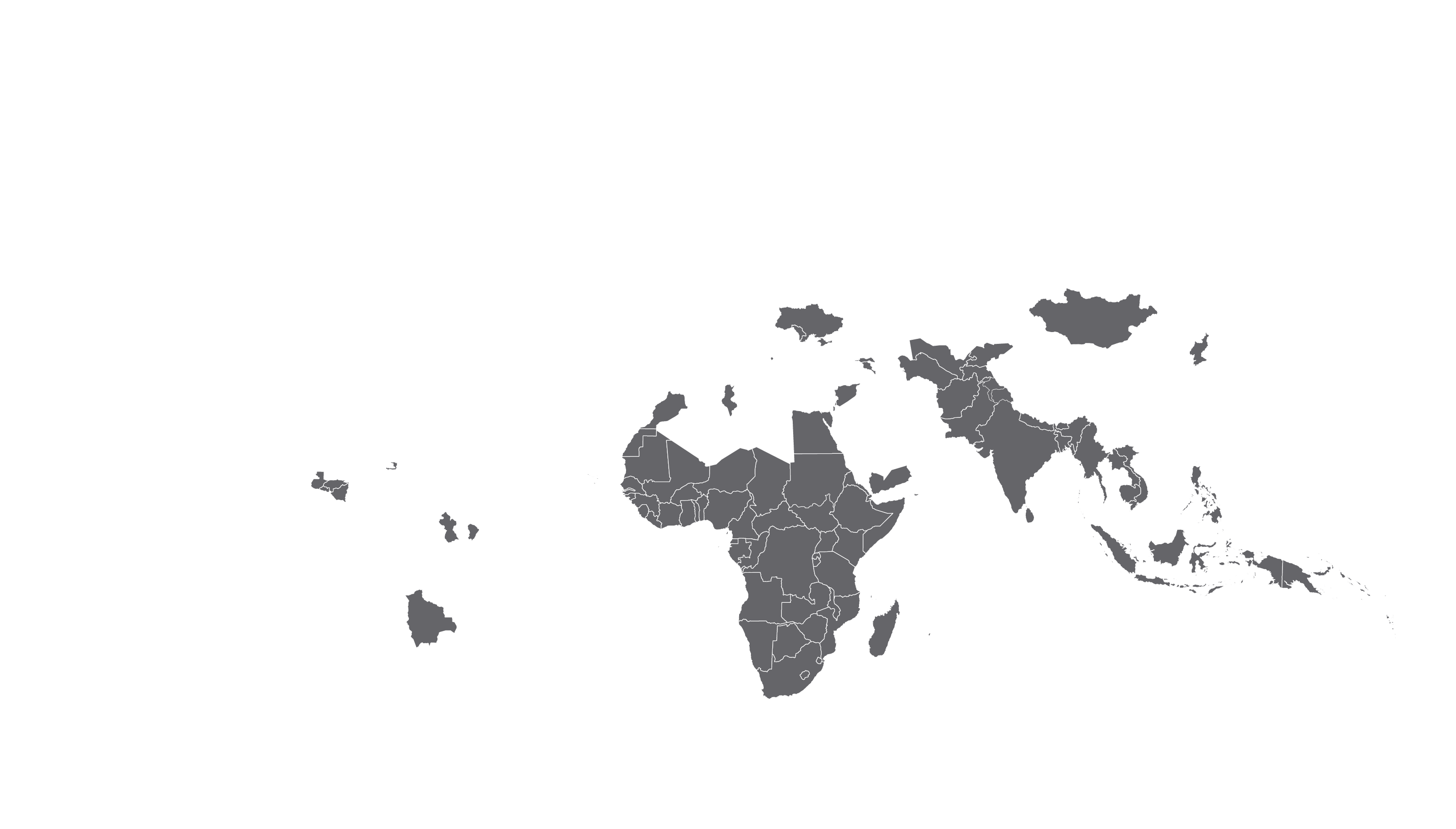
High-income countries
Low- and middle-income countries
COVERED TERRITORY
94
COUNTRIES
FILED IN
23
COUNTRIES
APPROVED IN
19
COUNTRIES
SOLD IN
27
COUNTRIES
High-income countries
Low- and middle-income countries
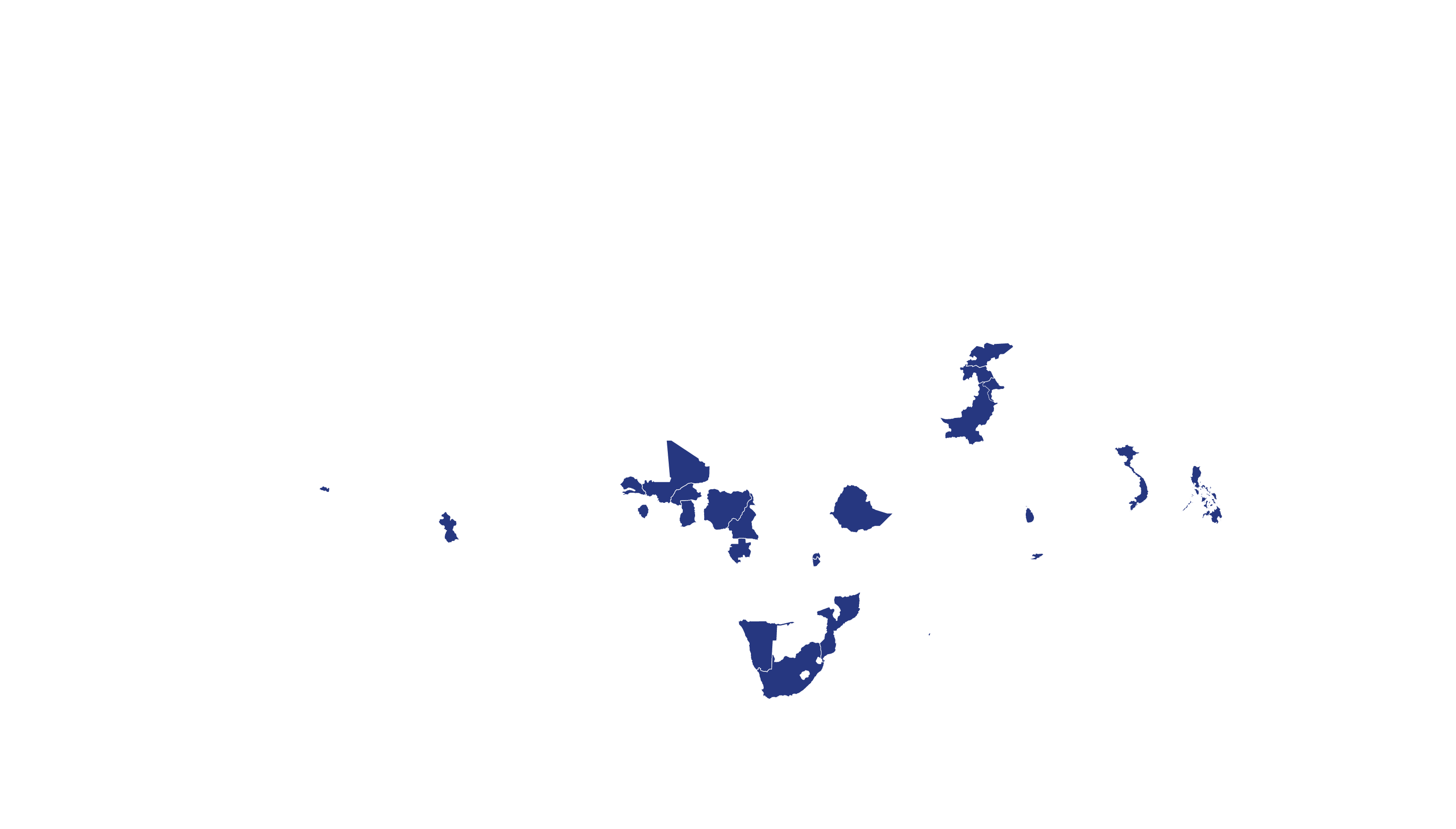
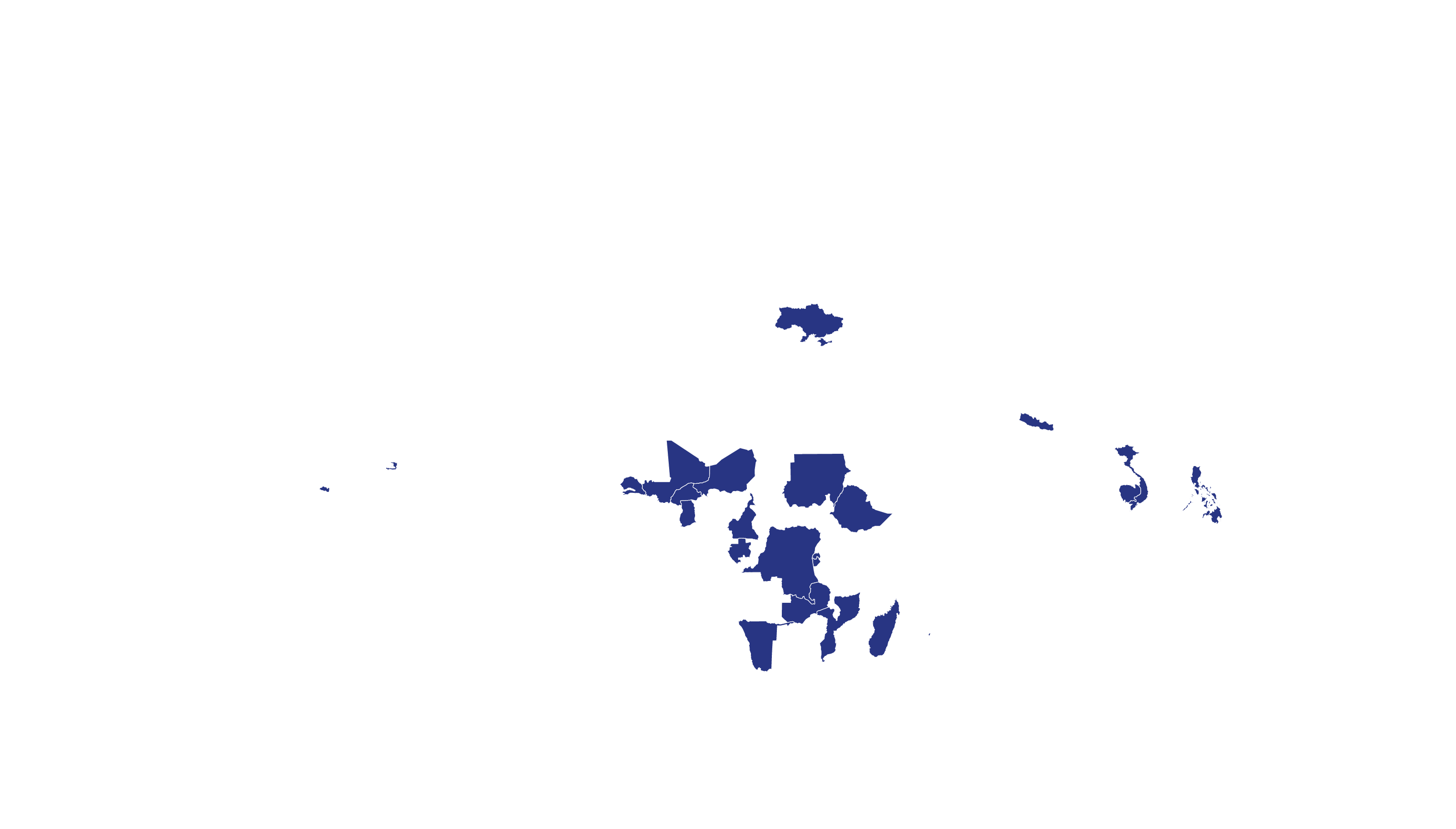
COVERED TERRITORY
87
COUNTRIES
FILED IN
8
COUNTRIES*
APPROVED IN
4
COUNTRIES
High-income countries
Low- and middle-income countries
*For confidential purposes, the list of filed countries will
be disclosed when more than one approval from stringent
regulatory authorities is granted
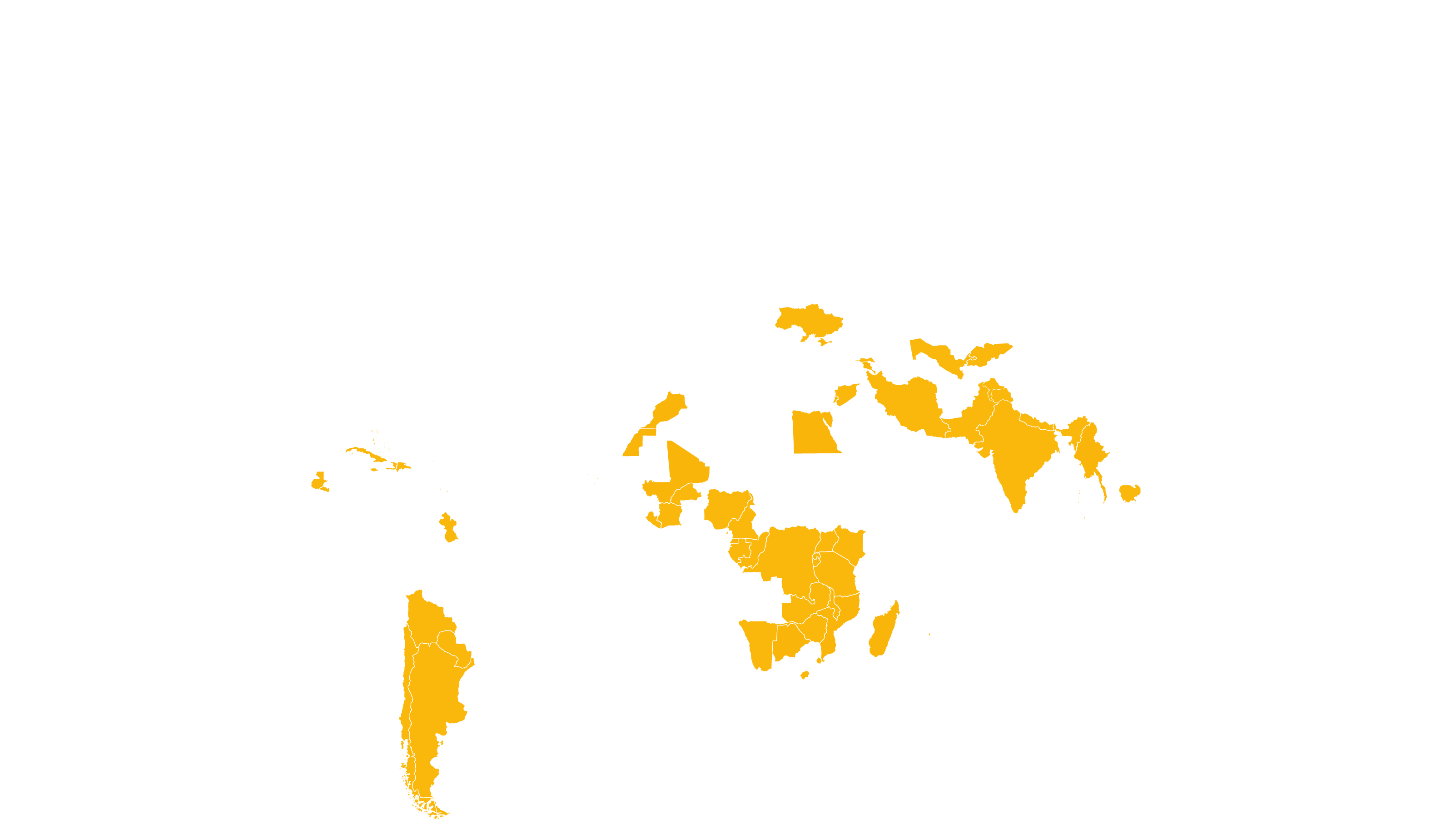
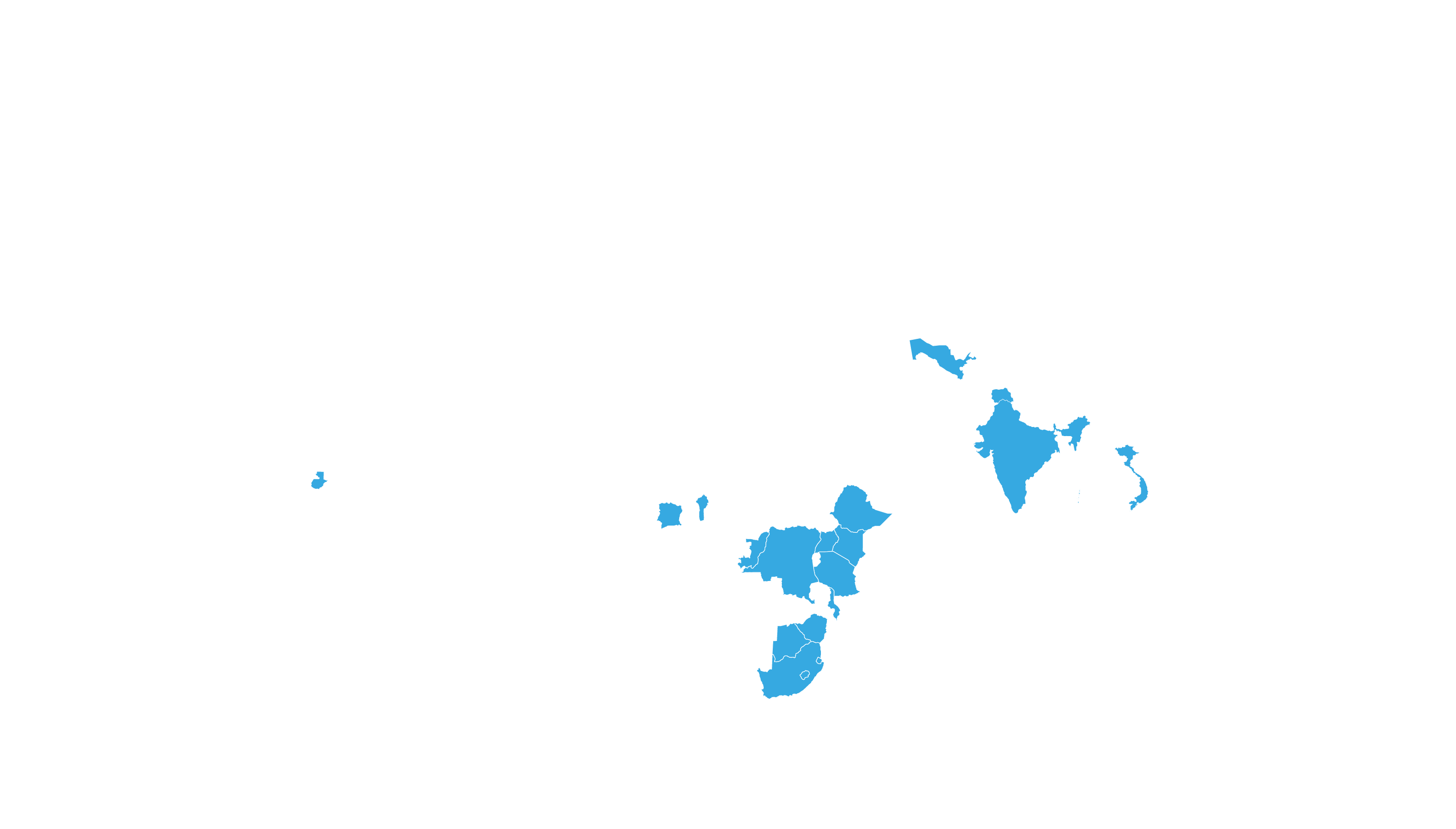
COVERED TERRITORY
122
COUNTRIES
FILED IN
12
COUNTRIES
APPROVED IN
35
COUNTRIES
SOLD IN
83
COUNTRIES
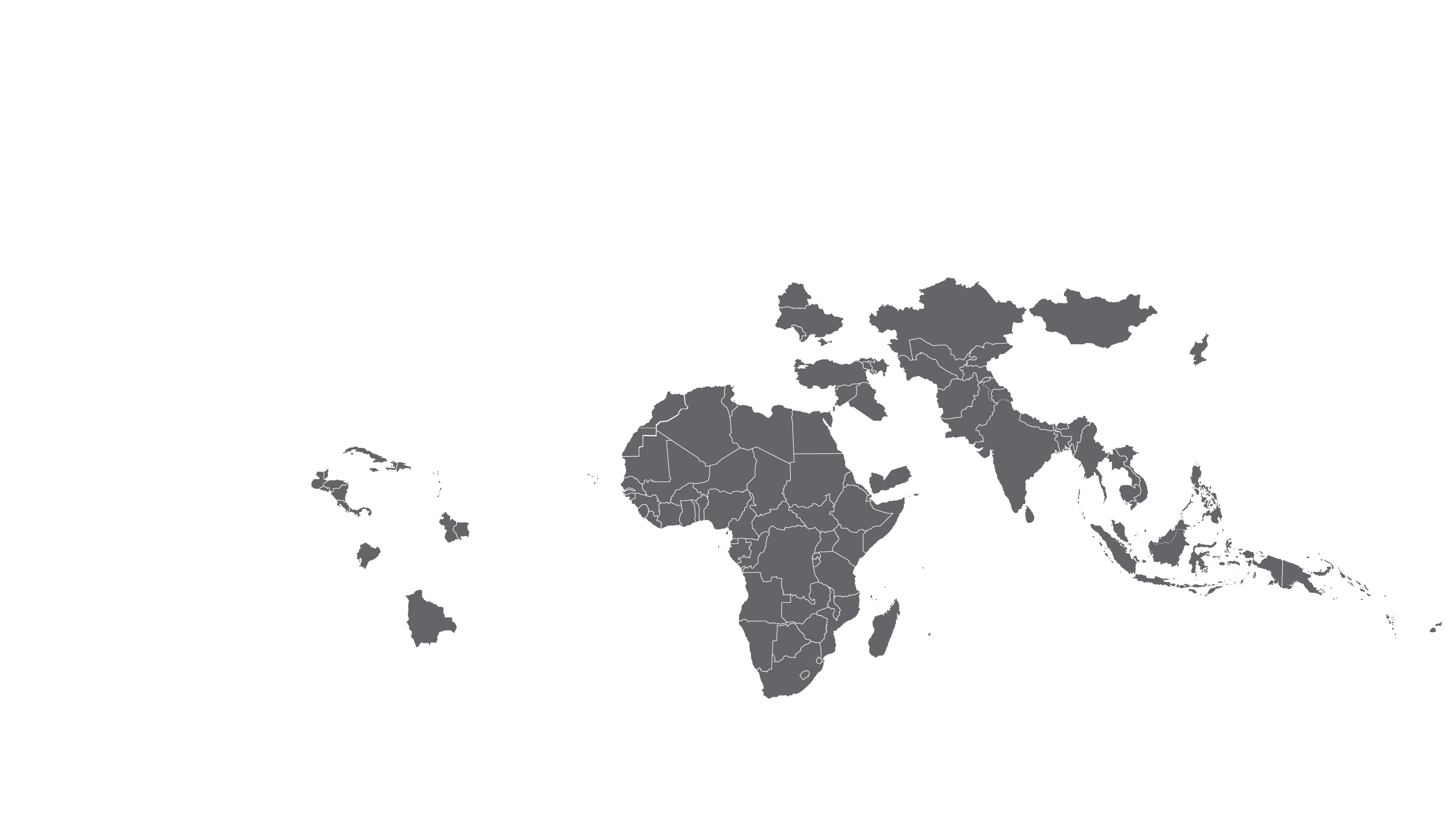
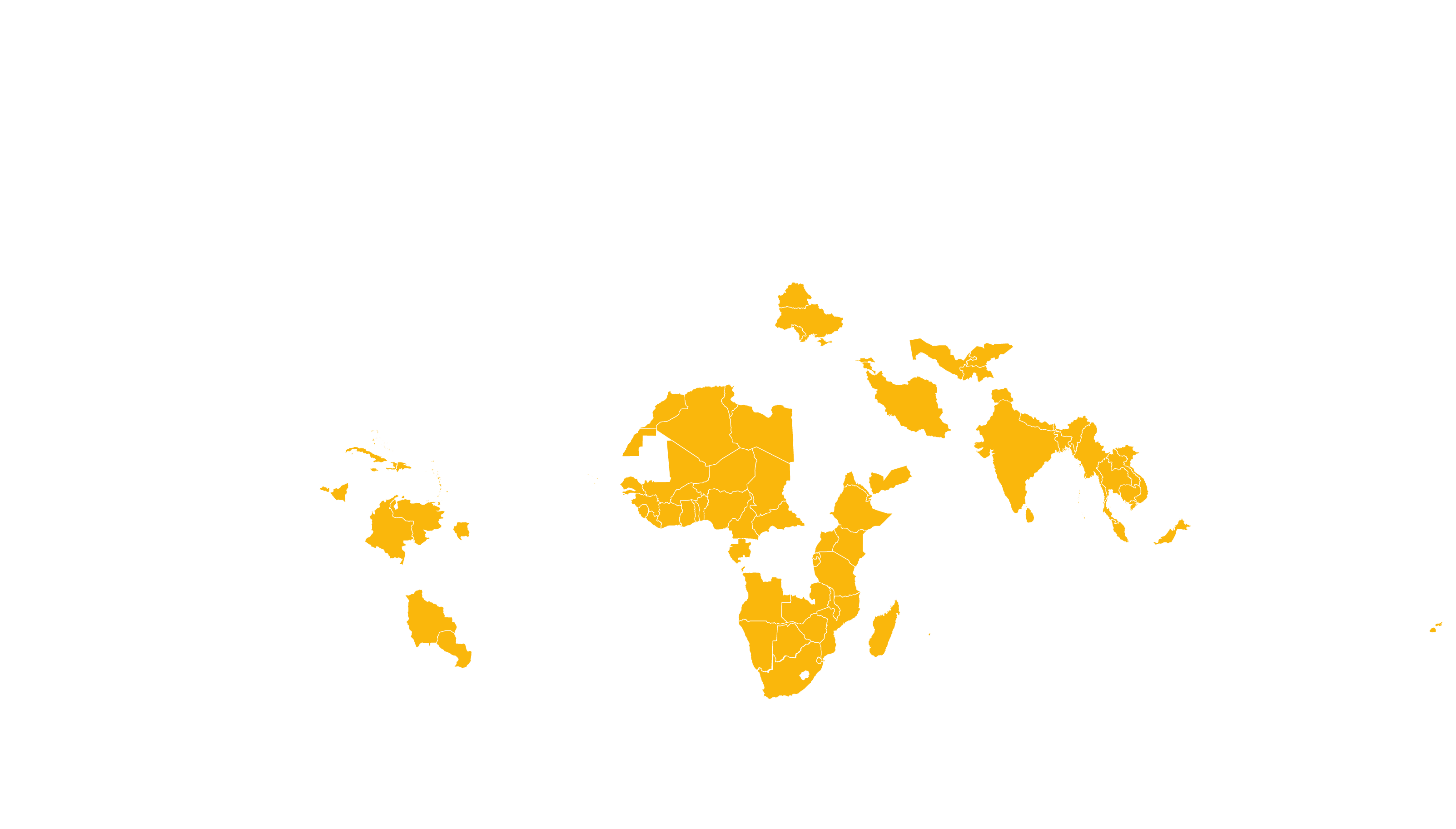
High-income countries
Low- and middle-income countries
COVERED TERRITORY
122
COUNTRIES
FILED IN
4
COUNTRIES
APPROVED IN
33
COUNTRIES
SOLD IN
70
COUNTRIES

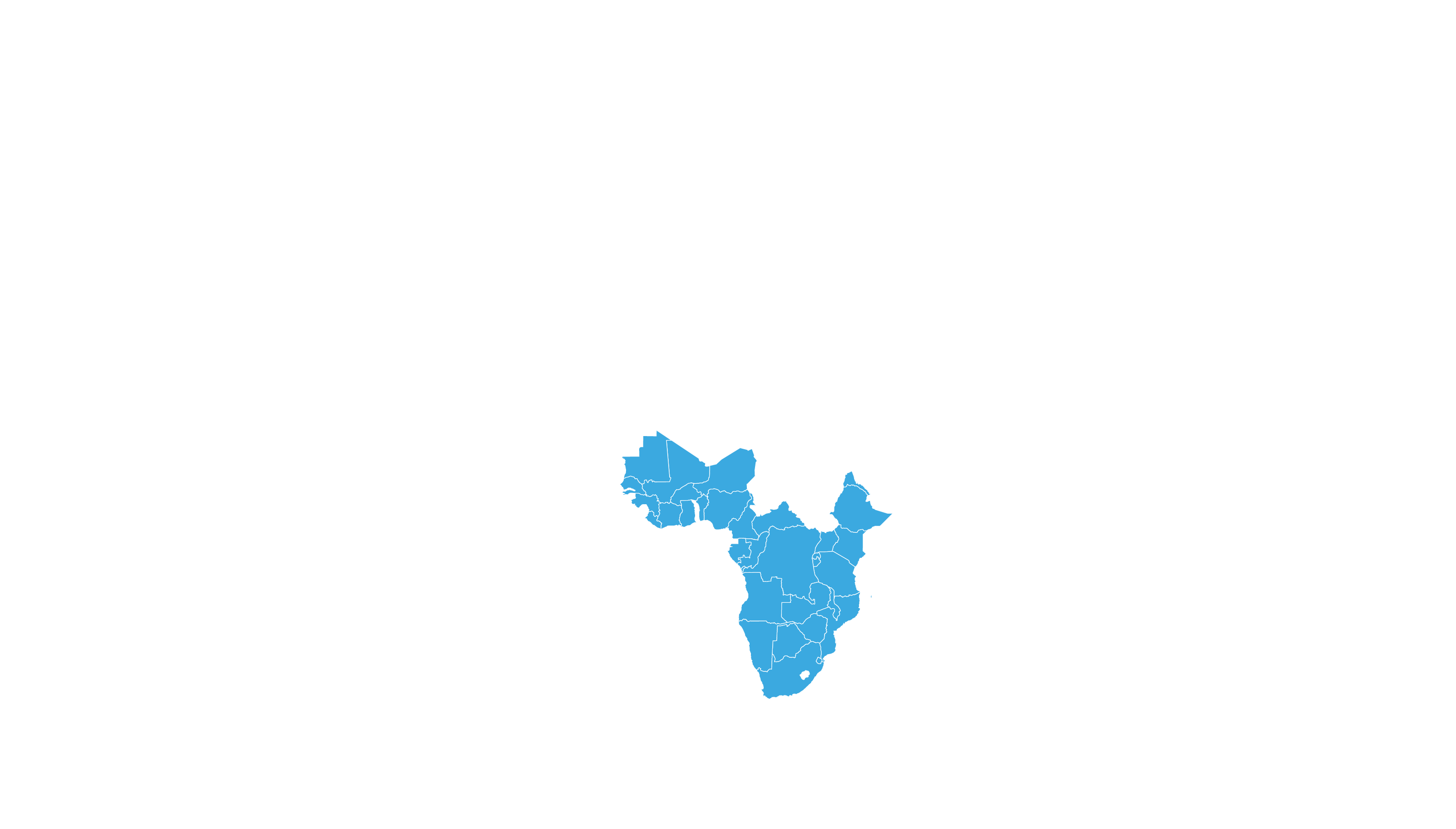
High-income countries
Low- and middle-income countries
COVERED TERRITORY
102
COUNTRIES
FILED IN
10
COUNTRIES*
APPROVED IN
9
COUNTRIES
High-income countries
Low- and middle-income countries
*For confidential purposes, the list of filed countries will
be disclosed when more than one approval from stringent
regulatory authorities is granted
Direct-acting antiviral medicines can cure more than 95% of patients
but access to diagnosis and treatment is low especially in low- and
middle-income countries, where the vast majority of people with the virus
live.2
Direct-acting antiviral medicines can cure more than 95% of patients
but access to diagnosis and treatment is low especially in low- and
middle-income countries, where the vast majority of people with the virus
live. of 2.3 million since 2016 and up from 8 million in 2010.2
PEOPLE HAVE
CHRONIC HEPATITIS C INFECTION

2015
20%
LIVING WITH
HEPATITIS C VIRUS
(HCV) INFECTION
KNEW THEIR
DIAGNOSIS
2015-2016
13%
STARTED
ON
TREATMENT
THERE IS STILL
A MAJOR GAP
TO ACHIEVE
THE 80% TREATMENT TARGET BY 2030.
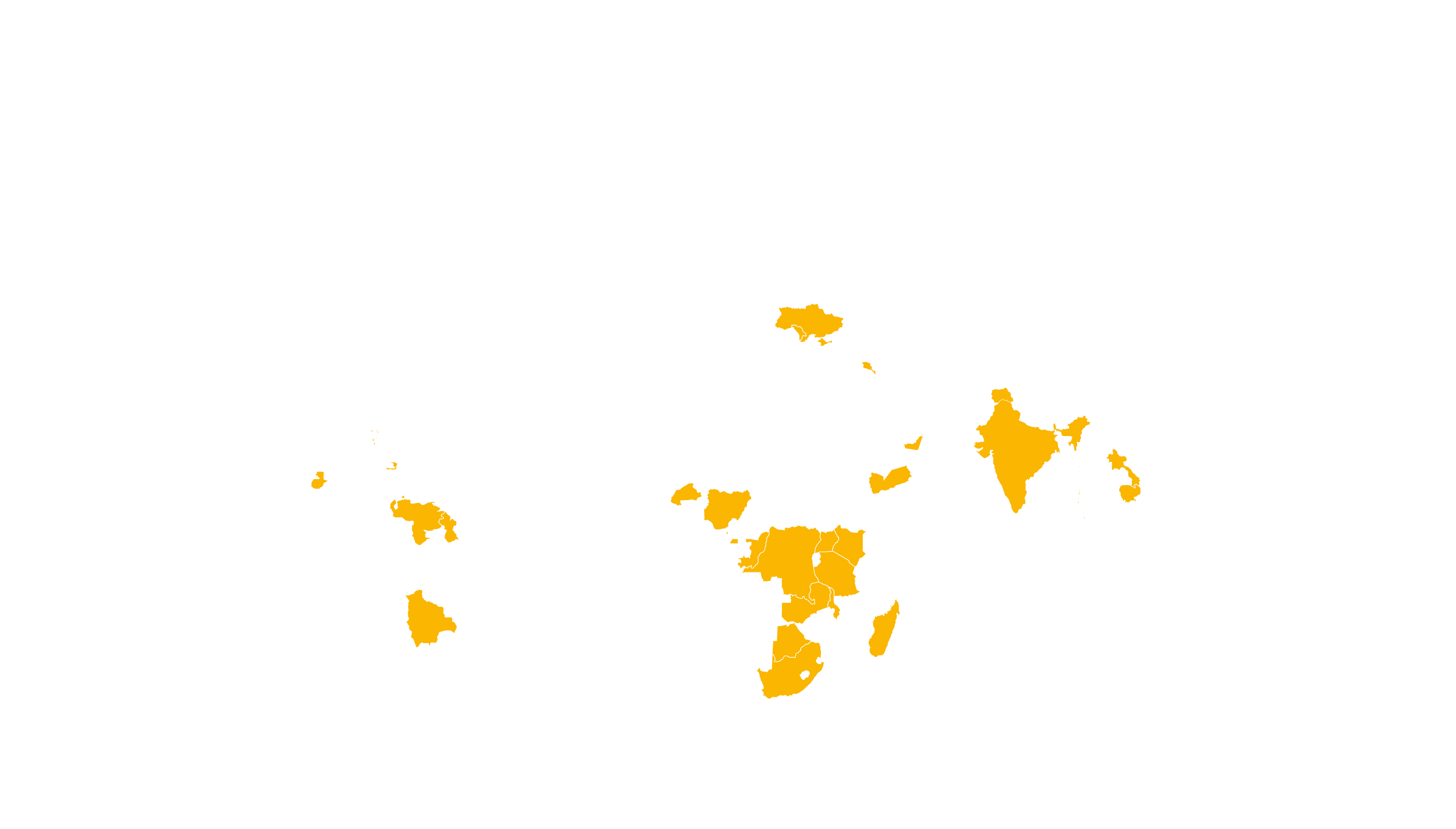
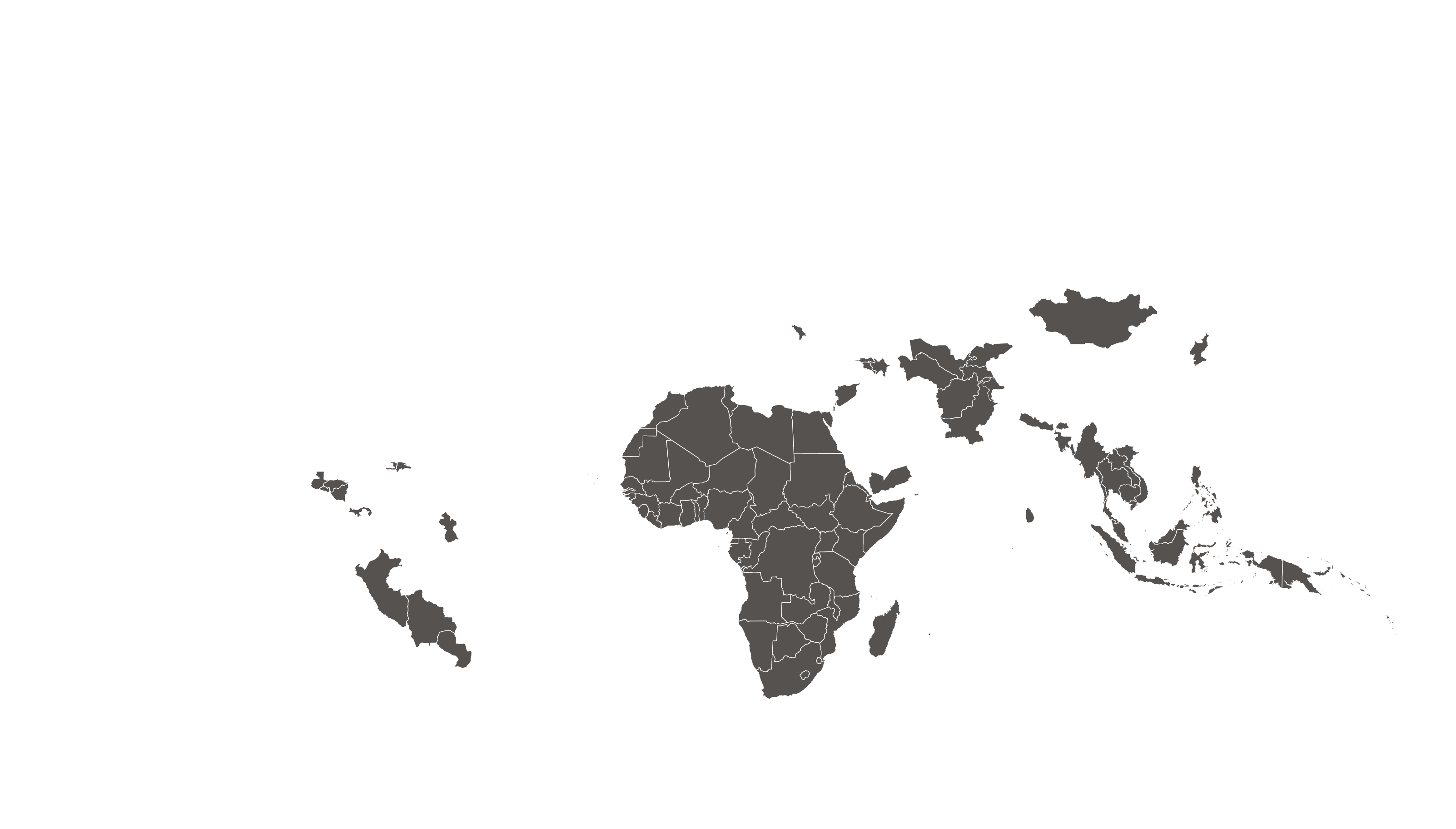
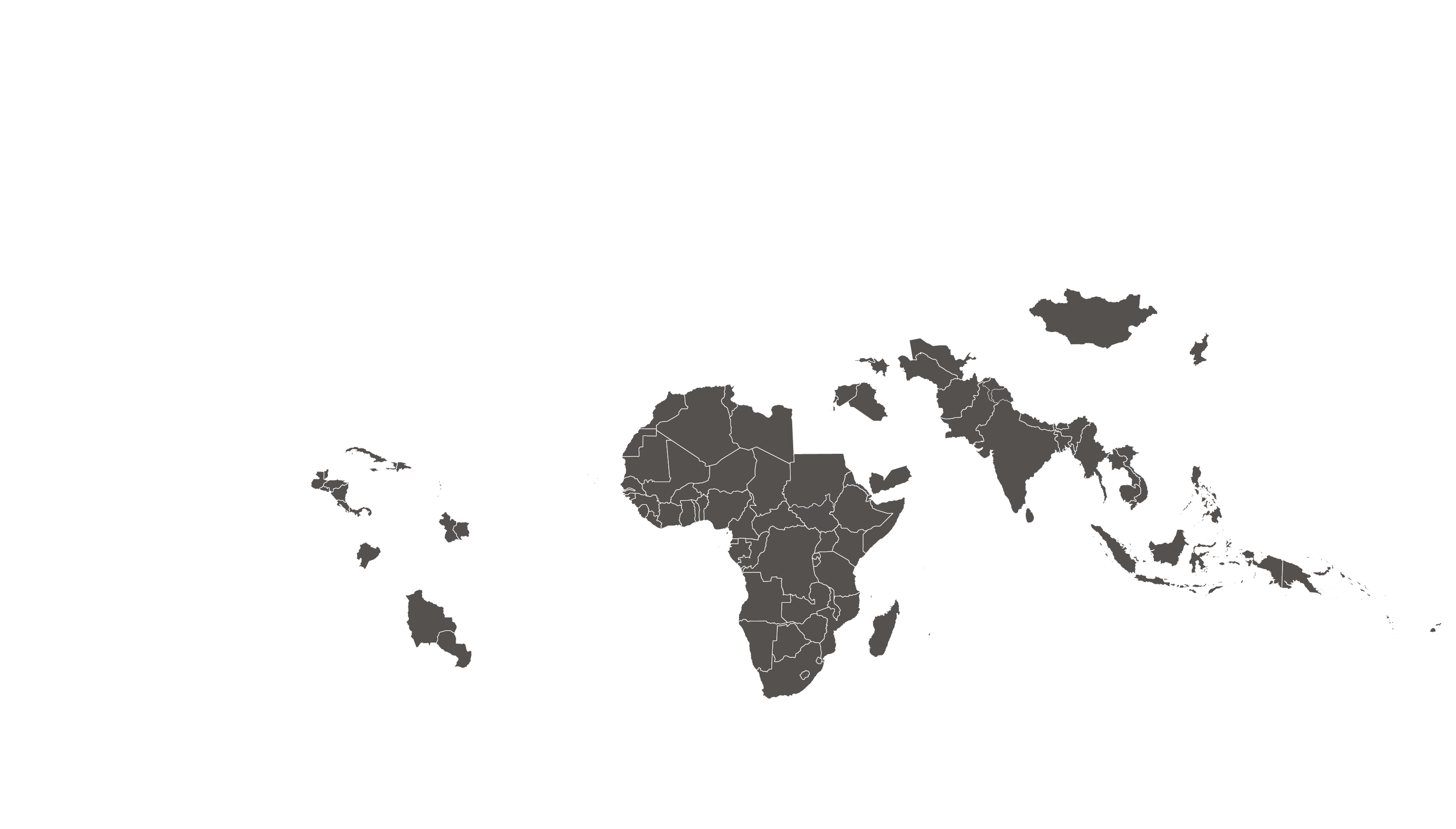
See how MPP's work is increasing access to Hepatitis C medicines in low- and middle-income countries - world map visualisation.
Choose product:
daclatasvir 30mg and 60mg
daclatasvir + sofosbuvir (DAC + SOF)
Choose status:
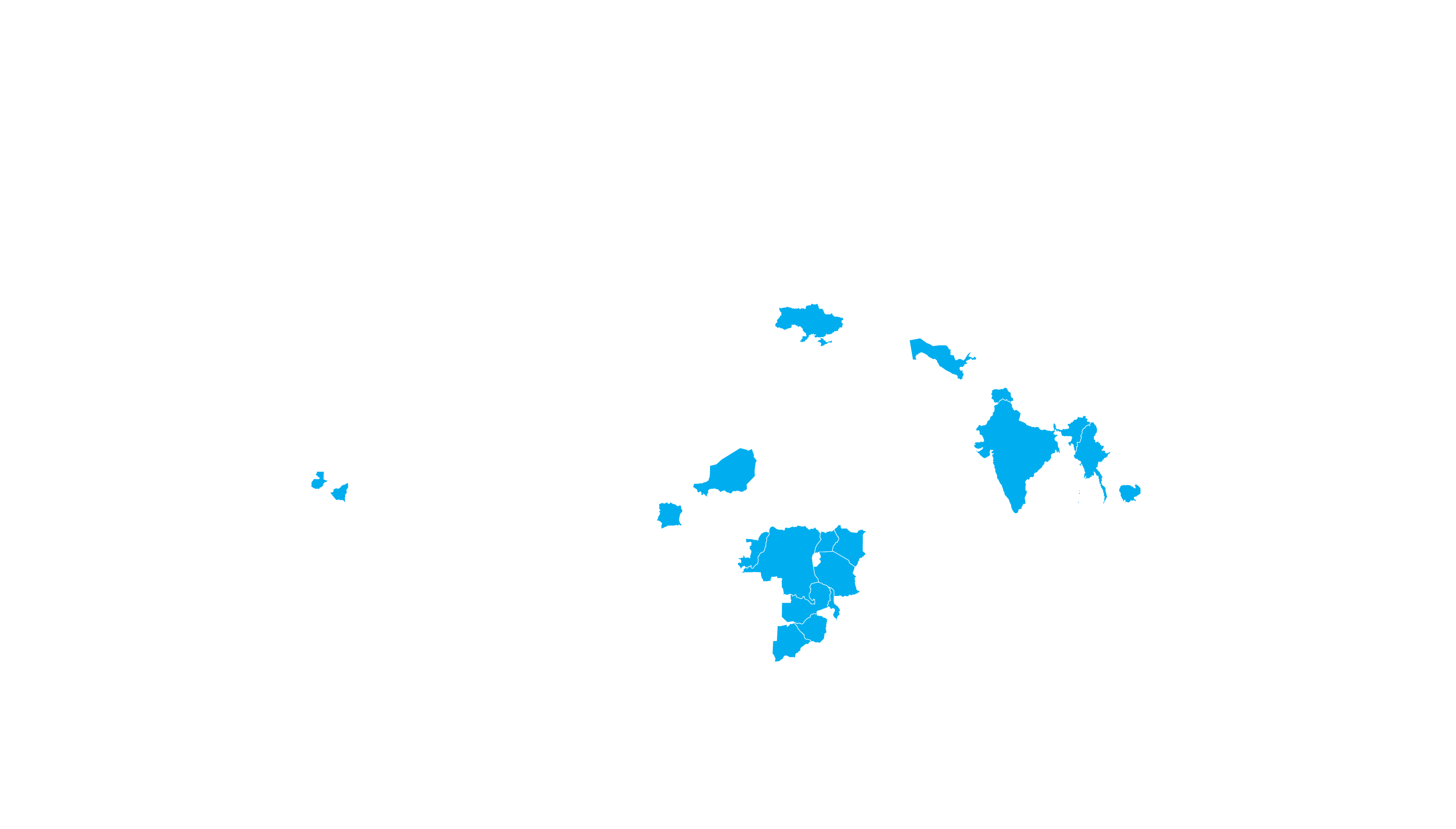
COVERED TERRITORY
112
COUNTRIES
FILED IN
29
COUNTRIES
APPROVED IN
25
COUNTRIES
SOLD IN
13
COUNTRIES
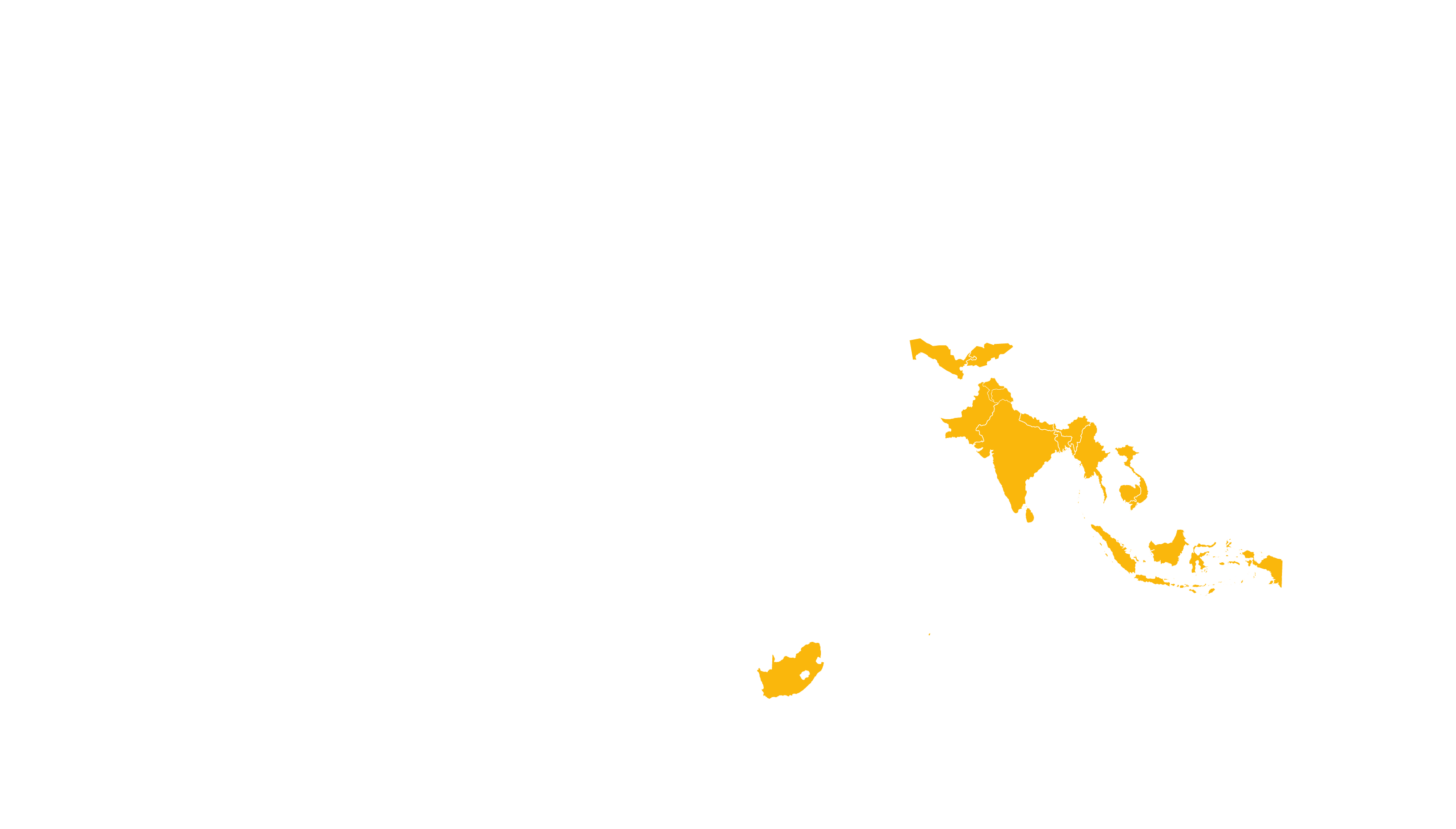
High-income countries
Low- and middle-income countries
COVERED TERRITORY
112
COUNTRIES
FILED IN
16
COUNTRIES*
APPROVED IN
5
COUNTRIES
SOLD IN
2
COUNTRIES

High-income countries
Low- and middle-income countries
*For confidential purposes, the list of filed countries will
be disclosed when more than one approval from stringent
regulatory authorities is granted
TB is one of the top ten killers globally and the leading killer of HIV-positive people.3
TB is one of the top ten killers globally and the leading killer of HIV-positive people.3
FELL ILL WITH
TB IN 2017

2017
87%
OF NEW TB CASES
OCCURRED IN THE
30 HIGH TB BURDEN COUNTRIES*
DIED
including
230,000 children
Ending the TB epidemic
by 2030 is amongst the health
targets of the Sustainable
Development Goals.
To meet this target, faster-acting,
better therapies to treat TB are urgent,
particularly for MDR-TB
Download
this chapter
In 2018, the MPP continued to support efforts to accelerate access to high-quality low-cost medical treatments and health technologies through the development of new tools and publication of reports.
MPP-Unitaid report released on long-acting technologies
Interactive antiretroviral projections tool launched
Annual Prioritisation Report
The Medicines Patents and Licences Database
Download
this chapter
for the year ended 31 December 2018 and Report of the Statutory Auditor